The Nanointerface Engineering Group at the University of Utah aims to gain a molecular-level understanding of important interfaces in materials science, catalysis, and medicine. We are an interdisciplinary group that brings together the fields of chemical engineering, chemistry, materials science, and medicine to produce innovative materials.
Current areas of focus
Biologically-inspired tailoring of heterogeneous catalysts
Taking inspiration from enzymatic systems that are able to fine tune their activity by manipulating the environment around the active site, our group designs, builds, and tests catalytic materials where we have carefully tailored the nanoenvironment around the active sites. We take a multi-scale approach to our nano-engineering of heterogeneous catalysts.
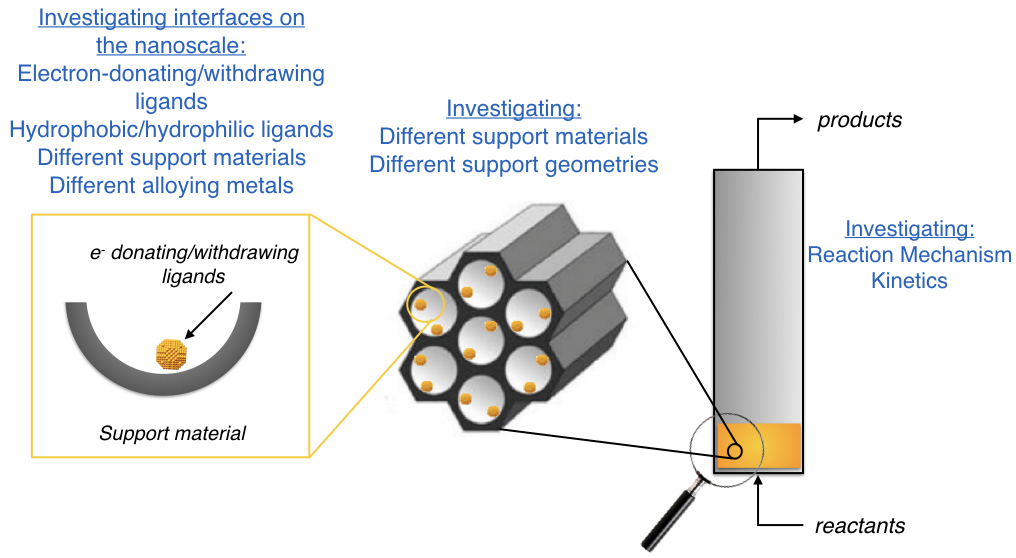
On the nanoscale, there are many interfaces that we are interested in tuning. These interfaces include metal-metal interactions (in multi-metallic clusters), metal-support interactions, and metal-ligand interactions. Each of these interfaces is important and provides an opportunity to tune the activity/selectivity of the active site.
On the mesoscale, we utilize different oxide support materials and investigate the behavior of our clusters when they are located inside or outside of pores. We use the pore geometry and surface chemistry to tune the environment around the active site.
On the macroscale, we investigate the catalytic performance of our materials in batch and continuous operation where the activity, selectivity, and stability of the catalyst are measured with in-situ characterization techniques.
Currently, work in the Nanointerface Engineering group involves using gold and gold alloy clusters for oxidation catalysis as well as novel structured Fischer-Tropsch catalysts.
Hybrid enzyme-metal nanoparticle cascade reaction systems
Natural systems are extremely successful in their application of cascade reactions, particularly in plants and animal species. Photosynthesis and the Krebs cycle are two examples of multiple enzymes working together to produce products and/or energy necessary for life. Enzymes are some of the most efficient and selective catalysts known and can catalyze chemical reactions under relatively mild conditions of low temperature, pressure, and reactant concentrations as compared to the high temperatures and pressures commonly used in industrial catalysis. An interesting and emerging area of catalysis is combining enzymes with metal nanoparticle catalysts.
The Nanointerface Engineering group is currently researching methods of synthesis as well as new catalytic applications of these hybrid enzyme-metal nanoparticle systems. As with the materials described above for heterogeneous catalysis applications, there are interesting interfaces that can be tuned to improve our catalysts’ activity and selectivity. The interfaces that are formed between the metal nanoparticle and the enzymes are of particular interest. How do these interfaces influence the performance of these materials in cascade reactions is a fundamental question for our research group.
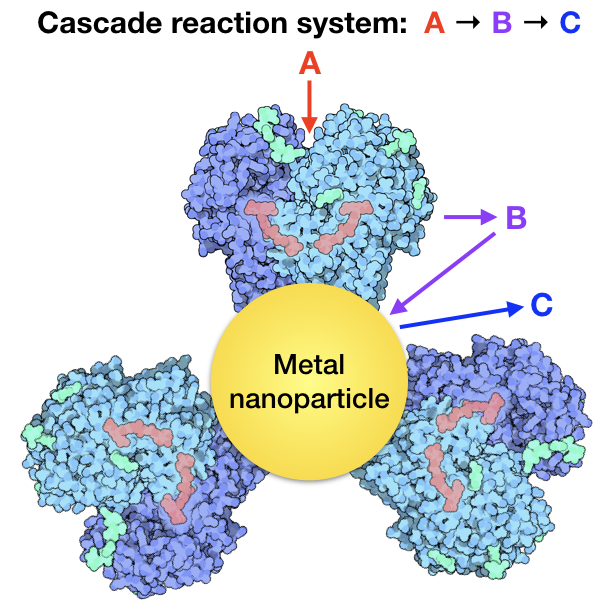
Current research in the Nanointerface Engineering group investigates oxidase-bound metallic nanoparticles for cascade reaction systems.
